Written by: Jens Lund, BSc in Biochemistry and bachelor in Nutrition and Health
We saw in a previous article how the proteins in the diet are digested, absorbed and utilized in protein synthesis. By extension, it is natural to also review the digestion and metabolism of carbohydrate and fat.
In this article, we will therefore take a closer look at what happens in the gastrointestinal system and later in the body's cells with the carbohydrates, we consume through the diet. Having a basic insight into these processes can include be beneficial to the understanding when the discussions on various training sites reach a slightly higher theoretical level. To limit the scope of the article, it will not address dietary fiber as well as their physiological and disease-preventing effects.
What is carbohydrate?
Carbohydrates are substances that consist of carbon (carbon), oxygen (oxygen) and hydrogen (hydrogen). The name carbohydrates is due firstly to the fact that they contain carbon and secondly to the fact that hydrogen and oxygen are included in the same ratio as in water - 2: 1. The carbohydrates in the diet are divided according to their size into monosaccharides, disaccharides, oligosaccharides and polysaccharides. Below you can see some of the most important carbohydrates in the diet, as well as what they consist of.
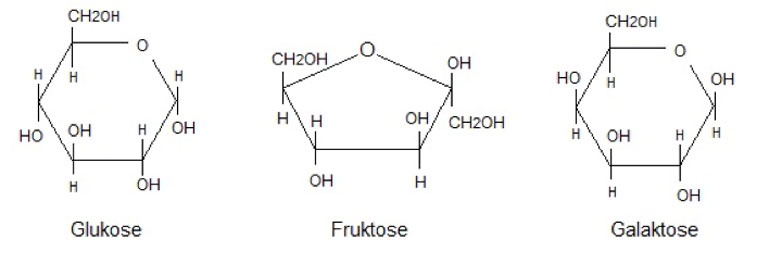
Monosaccharides - Consists of one sugar molecule (hexose)
- Glucose
- Fructose
- Galactose
We see that the monosaccharides are so-called hexoses. The term hexose simply refers to the fact that they contain 6 carbon atoms. Free galactose is rarely found in foods, while glucose and fructose are mainly found in fruits, berries, juices and certain vegetables. Glucose is physiologically the most important carbohydrate, as all the body's cells can utilize glucose, and when you talk about the level of blood sugar, it is also the blood content of glucose that is mentioned. Galactose is found in the milk of mammals, as the galactose in the mammary glands is put together with glucose to form lactose.
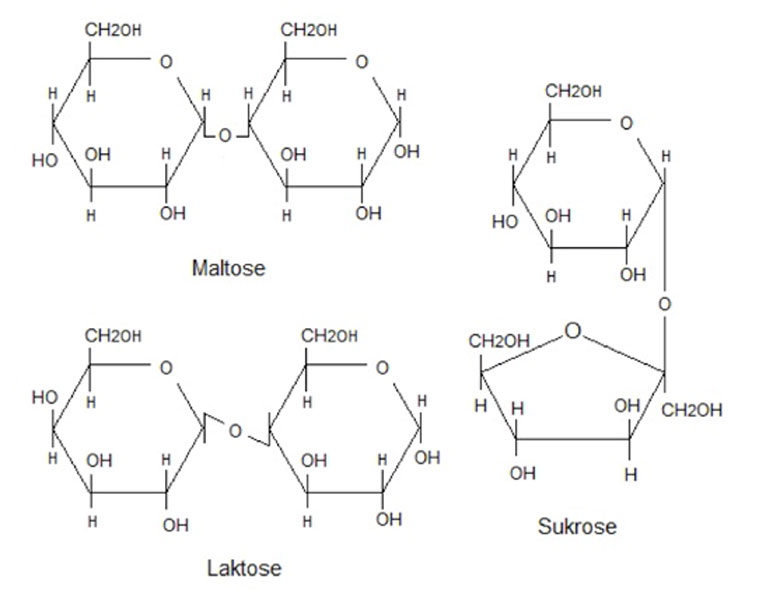
Disaccharides - Consists of two sugar molecules (hexoses)
- Maltose (Glucose + glucose)
- Lactose (Glucose + galactose)
- Sucrose (Glucose + Fructose)
The 3 disaccharides are also called malt sugar, milk sugar and cane sugar, and as you can see, they are composed of two monosaccharides. Sucrose is what we know as plain white table sugar and is found added in a sea of foods. Lactose is found in milk and dairy products and maltose is found e.g. in beer.
Oligosaccharides - Consists of 3 - 9 sugar molecules (hexoses)
- For example , maltodextrin , which is made up of glucose
Polysaccharides - Consists of> 10 sugar molecules (hexoses)
- Starch, which is made up of glucose
- Cellulose (dietary fiber which is also made up of glucose)
Both starch and cellulose are made up solely of glucose, but despite this, only the starch we can digest. This difference in digestibility is due to differences in the structure of the two substances. In starch, the glucose molecules are put together using alpha bonds, while in cellulose they are beta bonds. The body does not form an enzyme that is able to break down the beta bonds in cellulose, which is why cellulose is an indigestible carbohydrate.
The digestion of carbohydrate
Only the monosaccharides are small enough to be absorbed into the intestinal wall. The purpose of the digestion of disaccharides, oligosaccharides and polysaccharides is therefore to break them down into monosaccharides.
The digestion of carbohydrate starts in the mouth, where saliva is secreted from 3 salivary glands. Saliva contains the enzyme salivary amylase, which initiates the breakdown of starch. The salivary amylase cleaves the long chains of glucose into smaller chains to form oligosaccharides (dextrins). However, as the food only stays in the oral cavity for a short time, not much starch is digested in the mouth.
In the stomach, the salivary amylase cannot function at the low pH. However, some starch will still be digested, as the food in the upper part of the stomach will be placed in a suitable way that protects the salivary amylase with the low pH.
In the duodenum, the digestion of starch continues. A secretion containing amylase and the basic substance bicarbonate is secreted from the pancreas. Bicarbonate raises the pH so much that amylase can resume the breakdown of starch into maltose.
The last part of the carbohydrate digestion takes place in the small intestine using the 3 carbohydrate-breaking enzymes maltase, lactase and sucrose. These 3 enzymes break down disaccharides into monosaccharides. Maltase breaks down maltose into 2 glucose molecules, lactase breaks down lactose into glucose and galactose, and sucrose breaks down sucrose into glucose and fructose. After digestion in the small intestine, the monosaccharides are taken up through the wall of the small intestine and carried into the bloodstream.
The uptake of monosaccharides
After digestion in the small intestine, the monosaccharides are taken up through the wall of the small intestine and carried into the bloodstream. Glucose and galactose are absorbed very efficiently using a transport protein called GLUT2.
Part of the fructose is converted in the intestinal epithelial cells into lactate and glucose, while the rest is taken up by the transport protein GLUT5. From the intestinal epithelial cells, the monosaccharides are taken up into the venous blood vessels that surround the intestinal epithelium and then passed through the hepatic portal to the liver.
The conversion of carbohydrate
In the liver, galactose and the remaining fructose are converted to glucose or burned directly in the liver. It is therefore almost exclusively glucose that is released from the liver and carried with the blood on to other organs and tissues.
Glucose is the body's preferred fuel and can be stored in the form of glycogen in the liver and muscles. Glycogen, like starch, is long chains of glucose molecules put together. However, the storage capacity is limited to approx. 100 give the liver and 300 - 400 give the muscles. It is very convenient to deposit glucose in the form of glycogen instead of free glucose. This is because the accumulation of glucose in the cells will cause an extreme absorption of fluid (osmosis), which would burst the cells to pieces. The osmotic effect is greatly reduced by depositing glucose in the form of glycogen. However, it is inevitable that for every gram of glycogen deposited in the muscle and liver cells, 3 grams of water are bound.
Liver glycogen is used to regulate blood sugar between meals. After a meal, a certain amount of glucose is deposited, which can then be consumed during the time when no sugar is absorbed from the intestines. If there is an excess of glucose after the glycogen stores have been filled, the rest can be converted into fat . Muscle glycogen is the primary source of energy in the muscles, and once glucose has entered the muscle, it cannot leave it again. In other words, it is forced to be burned.
However, the glucose in the muscles can be converted to lactate, which can then be released and carried to the liver, where it can be converted into glucose (the so-called Cori cycle).
Regulation of blood sugar
Blood sugar is usually between 4 and 6 mmol / L in healthy people, but can rise up to 8 mmol / L after a meal. This increase in blood sugar stimulates the so-called beta cells in the pancreas to increase their secretion of insulin. Insulin lowers blood sugar by promoting the glucose uptake of muscle and fat cells in particular. More precisely, insulin works by increasing the amount of transport proteins called GLUT4 in cell membranes. This enables the muscle and fat cells to absorb the glucose, which flows around the blood, thus preventing a completely uninhibited rise in blood sugar. Insulin also promotes the formation of glycogen in the muscles and fat in the fat cells, increases the glucose metabolism in most cells and prevents the liver from converting protein into glucose.
Between meals, blood sugar drops, as glucose is constantly used in i.a. the red blood cells and the nervous system. Hypoglycemia (too low blood sugar) is a condition in which the concentration of glucose in the blood is as low as 2-3 mmol / L. In addition to reducing the ability to work, which you have probably experienced, hypoglycaemia can also lead to unconsciousness, as the central nervous system is deeply dependent on glucose. It is therefore appropriate that a low blood sugar stimulates the alpha cells of the pancreas to secrete the hormone glucagon. Glucagon promotes the breakdown of glycogen in the liver (glycogenolysis), thus counteracting an excessive drop in blood sugar between meals. If the glycogen depot is completely empty, glucagon will promote the conversion of i.a. amino acids for glucose (gluconeogenesis) and fat burning.
Adrenaline, cortisol and Growth hormone also participates in blood sugar regulation. Their functions as well as insulin and glucagon are summarized below.
Burning of glucose
We know that 1 g of carbohydrate (glucose) provides energy amount of 17 kJ (4 kcal), but how does the body utilize the energy found in carbohydrate? We will take a closer look at this in the following.
The chemically bound energy found in a glucose molecule cannot be used by the body. Therefore, the energy must be "transferred" to the molecule adenosine tri-phosphate (ATP). The chemical energy in ATP can be utilized by the body for e.g. muscle contractions or in other cellular processes.
When the body needs energy for a process, a phosphate group in ATP is broken down, so that it is converted to adenosine di-phosphate (ADP) and free phosphate (P). By this cleavage of P, energy is released. However, the body has only a limited amount of ATP, which is why ATP must be constantly restored. And it is here in this restoration that the nutrients of the diet come into the picture.
The body's cells "extract" the chemically bound energy in glucose by means of a sea of chemical processes. It would be far too extensive to give a complete description of all these reactions, which is why we are simply looking at how energy conversion takes place.
Glucose starts by being part of the chemical reaction series called glycolysis. Glycolysis takes place in the cell fluid (the cytoplasm), and in the process, 1 glucose molecule is converted to 2 molecules of pyruvate, while 2 molecules of ATP are restored.
If there is not enough oxygen present in the cell, pyruvate will be converted to lactate. The lactate formed can either be carried to the liver and converted to glucose, or it can later, when there is again enough oxygen in the cell, be converted to pyruvate and then to acetyl-CoA to eventually enter the aerobic recovery. of ATP.
In the aerobic recovery of ATP, which takes place in the mitochondria of the cells, pyruvate is first converted to the substance acetyl-CoA (pronounced acetyl coenzyme A). Acetyl-CoA can then enter the so-called citric acid cycle, where carbon dioxide and hydrogen are gradually decomposed. Citric acid cycle is, as the name suggests, a cyclic process in which acetyl-CoA in the first step is coupled with oxaloacetate to form citric acid. Through cleavages of CO 2and hydrogen, oxaloacetate is recovered, and can then be recoupled with acetyl-CoA, whereby another cycle can be performed.
Hydrogen is also produced in the glycolysis and in the conversion of pyruvate to acetyl-CoA, and this hydrogen is used, together with the cleaved hydrogen from the citric acid cycle, to recover ATP. The cleaved hydrogen contains energy-rich electrons, which release their energy through the electron transport chain.
This released energy is used to pump protons (H + ) across the inner membrane of the mitochondria. In some places in the inner membrane, the protons can seep back into the mitochondria, and when this happens, ATP is restored using the enzyme ATP synthase.
Eventually, the now low-energy electrons will be united with H +and O 2 to form H 2 O. In total, the whole process can be written as follows, with for each glucose molecule being burned, approx. 30 ATP:
C 6 H 12 O 6 + 6 O 2 à 6 CO 2 + 6 H 2 O + energy (ATP)
Summary
Dietary carbohydrates are divided into monosaccharides, disaccharides, oligosaccharides and polysaccharides. However, only the monosaccharides are small enough to be absorbed in the small intestine. It is therefore necessary that the di-, oligo-, and polysaccharides be cleaved into monosaccharides in the digestive system.
In the mouth, the enzyme salivary amylase initiates the breakdown of starch into oligosaccharides and maltose. This cleavage continues in the stomach until all the salivary amylase is denatured and thus no longer active due to the low pH. In the duodenum, saliva is secreted from the pancreas. The pancreas contains i.a. amylase and the basic substance bicarbonate. The bicarbonate raises the pH so that amylase can complete the cleavage of starch to maltose.
Later in the duodenum, the enzymes maltase, lactase and sucrose are secreted, which are broken down respectively. maltose, lactose and sucrose. After this, the monosaccharides are absorbed through the small intestine and carried with the blood to the liver. In the liver, fructose and galactose are converted to glucose and any excess sugar is deposited in the form of glycogen.
It is mainly the two hormones insulin and glucagon that regulate blood sugar. Insulin secretion is increased after a meal to prevent excessive increases in blood sugar, while glucagon secretion is increased between meals to prevent hypoglycaemia.
Glucose is burned in the cells using various biochemical processes. First, glucose is converted to 2 molecules of pyruvate in the glycolysis, then the 2 pyruvate molecules are converted into 2 acetyl-CoA molecules, which can then enter the citric acid cycle. During the citric acid cycle, hydrogen atoms are cleaved, which are used in the electron transport chain to recover ATP.
 Having a basic insight into these processes can be beneficial for understanding when the discussions reach a slightly higher theoretical level
Having a basic insight into these processes can be beneficial for understanding when the discussions reach a slightly higher theoretical level
Literature:
Nordic Nutrition Recommendations 2004 - Integrating nutrition and physical activity, 4th Edition, 2004.
Nedergaard, Gustav: Human nutrition - Basic book in nutrition, 4th edition, Nucleus, 2006.
Schibye & Klausen: Human physiology - Rest and work, 2nd edition , FADL's publishers, 2005.
Nielsen & Springborg: under the skin - Anatomy and physiology, 2nd edition, Munksgaard Denmark, 2005.
Bremer, Jens: Biochemistry and molecular biology, 2nd edition, Nucleus 2005





